Angiogenesis in Infectious Disease
Prof. Michael Stürzl, PhD
with Andreas Bohrer, Dominika Gaus, M. Sc. and Vinay Murtadak, M. Sc.
Endothelial cells (EC) are often in contact with pathogens because they form the inner layer of the blood and lymphatic vessels. Often they are affected by this interaction. Our research focuses on viral pathogens that either infect or at least modulate the function of the endothelium. At the moment we work on HIV-1 and HHV-8 based questions. Although EC are not the actual target cells of HIV-1, this virus heavily disturbs the endothelial function. This can be explained by the exposure of EC to cytokines released from infected cells (T-cells and monocytes) and by direct interaction of viral proteins with the endothelium. Our group was able to show that HIV-1 infected T-lymphocytes release increased amounts of VEGF-A. This may induce vascular leakage and stimulate proliferation of vascular endothelial cells (Ascherl et al., 1999). Besides this we could also show that the Tat protein, a regulatory protein of HIV-1 secreted by infected cells, and TNF-alpha, a cytokine increased in sera of HIV-1-infected patients, activate synergistically the adhesion of leukocytes to EC. Because this is a common pathogenic parameter of AIDS-associated vascular diseases, this may contribute to the vascular damage (Matzen et al. (2004)). At the moment we focus on the impact of glucose metabolism on HIV-1 replication.
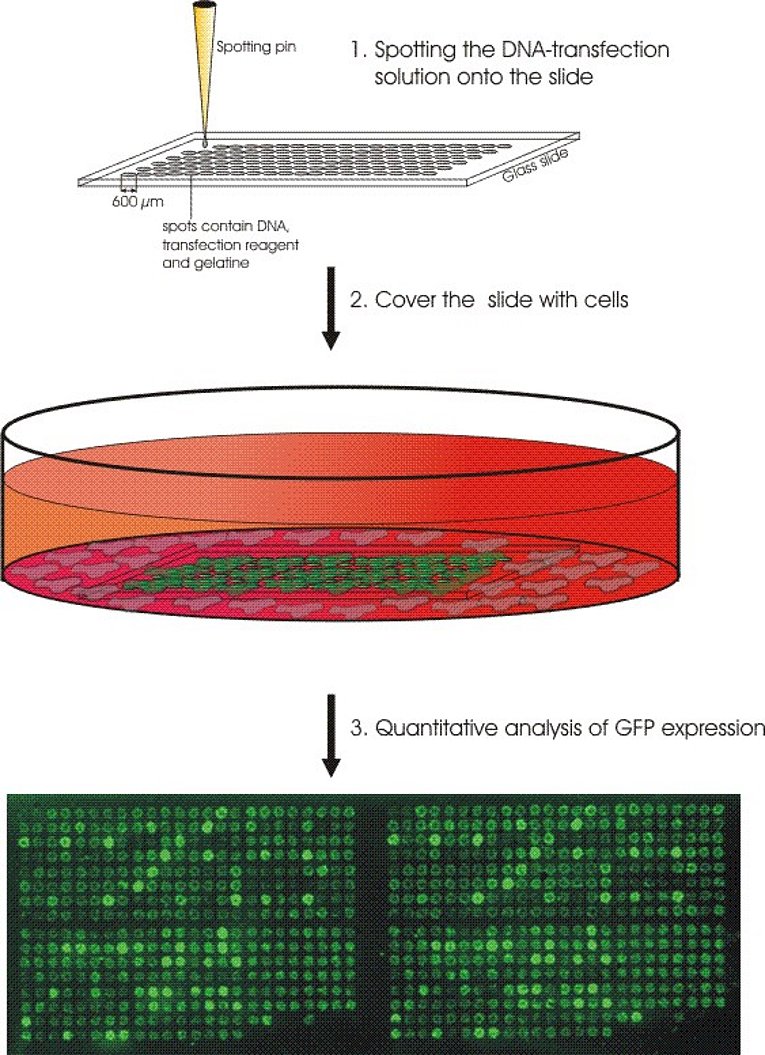
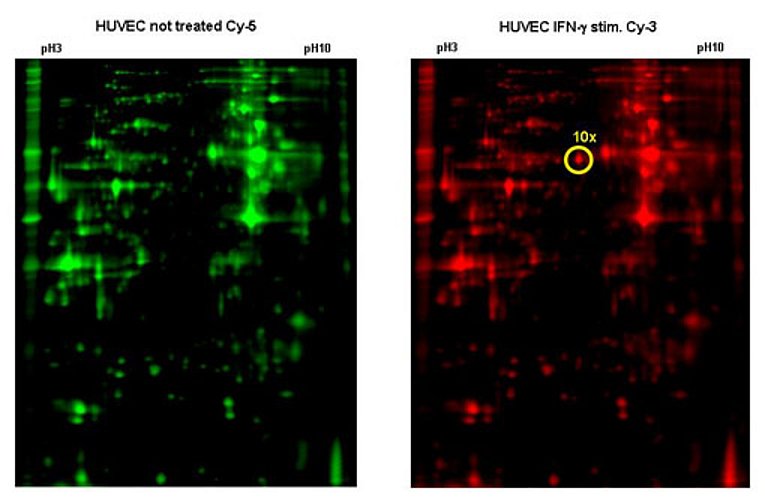
HHV-8 on the other hand directly infects EC. Under certain circumstances this can lead to Kaposi’s sarcoma (KS), one of the few tumours of endothelial origin. Using high-throughput approaches we try to identify HHV-8 genes that are important for the onset and development of KS. For this we established a method called reverse transfection array in the lab. With this method we can do several hundred transfections on a single glass slide in parallel (Fig. 2). We were able to show that some viral genes work in combination with each other. To test all possible pairwise combinations of all viral genes about 4000 transfections are necessary. In addition to this we try to find and characterize also cellular genes that play a role in the pathogenesis of KS. Therefore we generate primary EC constitutively expressing certain HHV-8 genes. Using DIGE technology (Fig. 3) and mass spectroscopy we aim to identify cellular genes that are specifically modulated by the viral genes.